
Feel free to contact Rachel at rneurath @ berkeley. edu if you have questions or comments regarding the content here.
Rachel Neurath arrived in my lab as a fully-formed Earth Scientist with a BA in Earth and Environmental Science from Smith College and an MS in Earth Science from Dartmouth. But she realized along the way that microorganisms were an important part of earth science about which she wanted to know more. So she came to Berkeley to expand her understanding of the microbial basis of Biogeochemistry. For the year before coming to Berkeley, Rachel taught High School Environmental Science in Falmouth MA. Her interest in mentoring flourished in ESPM as she became involved in Berkeley Connect and began to actively mentor undergraduate research projects in our lab. Rachel’s research has been recognized and supported by an NSF Dissertation Improvement Grant and a Lawrence Graduate Scholarship. Along the road to her PhD, Rachel and her husband Jesse had a beautiful daughter named Esme. This joy is well understood to make the challenging process of earning a doctorate substantially even more challenging. Rachel has persevered and will very soon become Dr. Rachel Neurath. I am immensely proud of her!
– Mary K. Firestone, Professor of the Graduate School, ESPM
About Rachel.
I am a soil microbial ecologist interested in carbon cycling and the impact of climate change on soil ecosystems. For my dissertation, I explore the mechanisms of carbon persistence in soil ecosystems. Advised by Dr. Mary Firestone, my research lies at the intersection of soil biogeochemistry and microbial ecology. I am particularly interested in plant-microbe-mineral interactions. Plant roots are the primary source of carbon in soils. If this carbon associates with soil minerals, it may persist in soils for thousands of years, storing carbon in the soil and offsetting atmosphere carbon. The fate of this mineral carbon is largely dependent on the composition and activity of the soil microbial community. I also explore the importance of mineral type and carbon source on the fate of mineral associated soil carbon. We are finding that plant-microbe-mineral interactions are complex and dynamic.
Dissertation title:
Plant-Microbe-Mineral Interactions Control Carbon Persistence in Soil
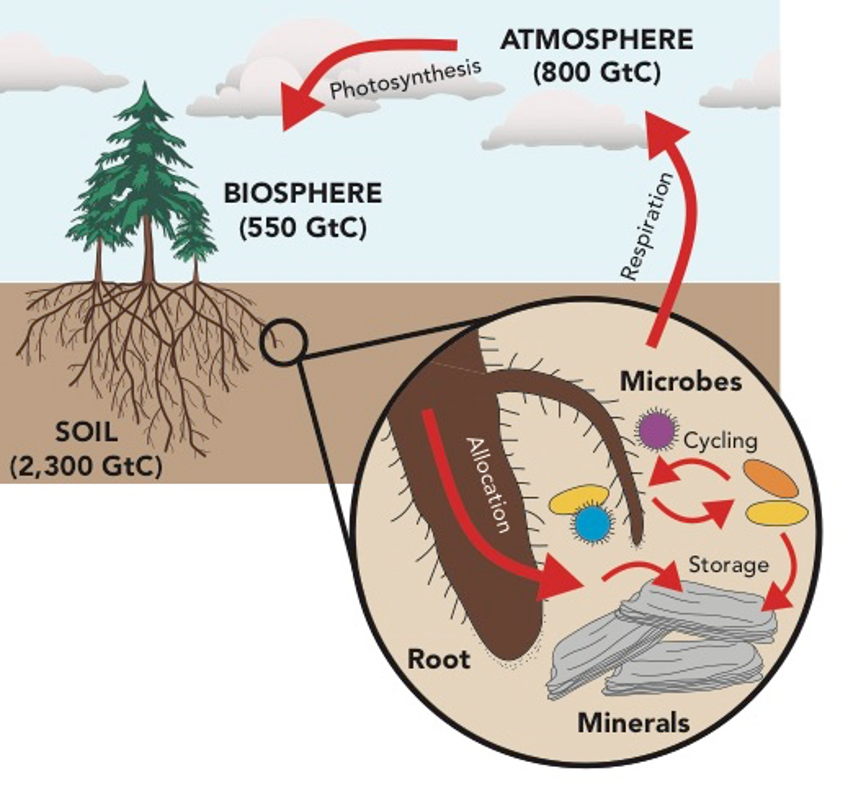
You may just call it dirt, but the soil beneath our feet is extraordinarily complex, teaming with life, and may just help us address climate change. Soils are the largest terrestrial carbon reservoir, storing more carbon than the biosphere and atmosphere combined. A history of destructive land management practices, including deforestation and intensive agricultural, caused soil carbon loss. Can we manage soils to increase carbon storage, removing carbon dioxide from the atmosphere? Perhaps, but first we need to better understand the mechanisms of soil carbon storage. My dissertation research explores how interactions between plants, microbes, and minerals influence the fate of carbon in soil.


Plants:
Plants are the primary carbon source in soil. Plants photosynthesize carbon dioxide from the atmosphere. A portion of that carbon is released into the soil as root exudates, small carbon compounds including sugars and organic acids. Photosynthesized carbon is also incorporated in plant biomass, and when the plant decomposes, a portion of that carbon enters the soil organic carbon pool. My research focuses on a slender oat grass, Avena barbata, common in northern California, with related species found across Mediterranean grasslands globally. A. barbata is an annual grass. It grows during the wet season in the fall and winter and senesces in late spring. During this growing season, carbon enters the soil primarily as root exudates, though roots are continually growing and old ones start dying off, so there is some decomposition, as well. In the summer, there is no growth, so the only contribution of carbon from A. barbata to the soil is from decomposing root and shoot biomass. The first two chapters of my dissertation focus on the growing season, looking specifically at “the rhizosphere”, or zone of root influence, while my third chapter examines what happens after the plant senesces, in what I call “the detritosphere”.

Microbes:
Soils harbor an incredible diversity of microorganisms. In a typical surface soil, a single teaspoon of soil contains a billion bacteria and a million fungi. These microbes control biogeochemical cycling in soil, including carbon cycling. A large fraction of plant-derived carbon in soil is taken up and transformed by soil microbes. That carbon may be released back to the atmosphere as carbon dioxide. Or it could continue cycling through the soil, occasionally persisting for up to thousands of years. Microbes are an important control on the fate of carbon in soil. Different microbes are able to take up different types of carbon. Microbes in the rhizosphere are a distinct subset of the overall microbial community, many with the ability to quickly metabolize root exudates. Among the microbial community surround the A. barbata rhizosphere are arbuscular mycorrhizal fungi, or AMF. The are attached to the root and reach thin fungal hyphae out into the soil, acquiring essential nutrients and water for the plant in exchange for carbon.
Minerals:
Soil minerals, which come from weathered bedrock, associate with soil carbon. When carbon associates with a soil mineral, it may come right back off, or it may persist. Radiocarbon dating of mineral-associated carbon shows that it can be thousands of years old (Torn et al., 1997, Nature). To store more carbon in soil, we not only need to get more carbon into the soil; we need it to stick around. Minerals may be an important key to increasing soil carbon storage. Carbon associates to minerals through chemical bonding. Microbes also colonize mineral surfaces, and both the microbes themselves and their byproducts, including biofilms, can become part of the mineral-associated carbon pool. When carbon is associated with a mineral, it less accessible to microbes. The type of mineral, along with soil characteristics like pH, determines the types of bonds that can occur between minerals and carbon. Some bonds are stronger than others. Thus, mineralogy is an important predictor of how long mineral-associated carbon persists in the soil. In my research, I compare three mineral types. Quartz has the lowest surface area and reactivity, kaolinite the highest surface area and moderate reactivity, and ferrihydrite has moderate surface area and high reactivity. I also look at a natural assemblage of minerals extracted from the soil using a technique called density fractionation.
Methods:
My dissertation research uses a technique called stable isotope tracing. Stable isotopes like the one I used, 13C, do not decay like radioactive isotopes. The isotope 13C occurs naturally in our atmosphere, but at a low concentration of 1% of all carbon isotopes. For my first two chapters, I grew A. barbata in a sealed plant growth chamber where we added CO2 gas with 99% 13C. As the A. barbata grew, it incorporated this labeled 13C. We were then able to trace incorporation of 13C into the soil to follow the fate of the plant-derived carbon. In my second chapter, I saved dried roots from my earlier work, and incorporated them into the soil as root litter. It was still labeled with 13C, allowing me to trace the fate detrital root litter.

To look at how much root-derived carbon associated with different mineral types, we measured total carbon on each mineral type and then I looked at how much of that carbon was 13C using isotope ratio mass spectrometry (IRMS). To map 13C incorporation and distribution in samples at a fine scale, I used nanoscale secondary ion mass spectrometry (NanoSIMS) as part of a four-year Lawrence Graduate Scholar fellowship at Lawrence Livermore National Laboratory, working with Dr. Jennifer Pett-Ridge. I also characterized the chemistry of mineral-associated carbon using 13C nuclear magnetic resonance mass spectrometry (13C-NMR), Fourier-transform ion cyclotron resonance mass spectrometry (FTICR-MS), and lipidomics in collaboration with the Environmental Molecular Sciences Laboratory (EMSL) at the Pacific Northwest National Laboratory. To examine fine-scale variation in mineral-associated carbon chemistry, I worked with Dr. Peter Nico at Lawrence Berkeley Laboratory on scanning transmission X-ray microscopy – near edge adsorption fine structure spectroscopy (STXM-NEXAFS) analysis. To study the mineral-associated microbial communities, we extracted DNA from the minerals and sequenced the phylogenetic markers 16S for bacteria and ITS for fungi using MiSeq high throughput sequencing.

Results and Discussion:
Plants, microbes, and minerals all interact to influence the fate of soil carbon. My dissertation only examined a single plant growing season for my first two chapters, and followed soil from a dry detrital system through a simulated wet-up (first rainfall) and the following three months in my third chapter. I see the initial stages of carbon association with soil minerals, using specimen minerals that started with no detectable carbon, compared with native minerals density fractionated from the soil. While more work is needed to follow the fate of mineral-associated carbon over multiple years, or, ideally, decades, our results offer some interesting insights into some of the mechanisms of carbon association – and disassociation – with minerals.
My dissertation shows that mineral type controls how much carbon associates with the mineral and how much of that carbon is plant-derived (Neurath et al., in prep). Mineral type also shapes microbial community colonization, which was structured by mineral type (Whitman, Neurath, et al., 2018). While the chemistry of mineral-associated carbon also did vary based on mineral type, presence or absence of growing plant roots was the dominant control on chemistry (Neurath et al., in prep). A large portion of mineral-associated carbon, particularly the carbon that appeared more persistent, was in the form of lipids. Lipidomic analysis showed that many of these lipids were microbially-derived (Neurath et al., in prep). In particular, many of these lipids were fungal. We know that AMF colonize the minerals (Whitman, Neurath, et al. 2018). In our NanoSIMS analysis, we find that a significant portion of plant-derived carbon on the minerals comes from AMF hyphae. We have some initial evidence that AMF may play a key role in carbon association with minerals in our study system (Neurath et al., in prep).
Carbon association with minerals is highly dynamic, perhaps particularly so in the rhizosphere. While carbon is continually associating with minerals, it also comes off. Minerals in the rhizosphere rapidly accumulated a chemically distinct carbon fingerprint, but it was largely transient. Lipids were one of the most persistent forms of carbon on the minerals. While mineralogy was the dominant control on carbon persistence, the source and form of carbon also influenced its fate (Neurath et al., in prep).
My dissertation research suggests that soils with more reactive mineral types, such as ferrihydrite, may have the potential to store more carbon. Lipids appear particularly persistent, and a large fraction are microbially derived, so while microbial metabolism causes carbon loss from soil, microbes also play a critical role in carbon transformation and persistence. My research is just one of many studies emerging in this exciting field, and there is more to learn before we can develop an ideal strategy for soil carbon storage. Still, I am optimistic that in many soils, increased soil carbon storage is possible.

Rachel’s dissertation research has been funded by the following agencies and awards
- Office of Science, Department of Energy
- Doctoral Dissertation Grant, National Science Foundation
- Lawrence Graduate Scholar Program, Lawrence Livermore National Laboratory
- Environmental Molecular Sciences Laboratory, Pacific Northwest National Laboratory
- Advanced Light Source, Lawrence Berkeley Laboratory


